Wan Nordiana Rahman1,2*, Noor Nabilah Talik Sisin1,2, Raizulnasuha Ab Rashid3
1Department of Applied Physics, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia.
2Nuclear Technology Research Center, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia.
3Centre for Diagnostic Nuclear Imaging, Universiti Putra Malaysia, 43400, Serdang, Selangor, Malaysia.
Introduction
Since the discovery of X-rays more than 100 years ago, ionizing radiation has been used extensively worldwide for medical imaging and treatment. The primary objective of cancer treatment using ionizing radiation has never really changed since then: to deliver the maximum dose to the targeted tumor while minimizing the radiation effects to the surrounding healthy tissues. The advancements in radiotherapy technologies are primarily developed to achieve this objective.
Despite that, the side effects of radiotherapy never ceased to persist. The exact localization of dose to the tumor remains a considerable hurdle to be surpassed due to external radiation delivery as it must pass through healthy tissues to reach the commonly deep-seated tumors. The radiation also scatters upon interaction, compromising the neighboring healthy tissues. In addition, some cancer patients are more radiosensitive than others. Therefore, around 5% of the patients received limited radiation dosage to avoid adverse side effects.
Multiple post-treatment remedies were used to treat these side effects. However, this option will introduce additional costs at the expense of the patient’s quality of life. Thus, it is essential to continue discovering ways to maximize the treatment efficacy and healthy tissue sparing in the current and upcoming radiotherapy treatment regimes. The effectiveness of stereotactic body radiation treatment (SBRT) in enhancing the biologically effective dose (BED) is unquestionable, as multiple clinical reports have already proven the superior outcome of cancer management by using SBRT over conventional therapies (Rosenberg et al., 2019). This favorable outcome entices the oncologists to escalate the radiation dose further to improve tumoricidal effectiveness. However, this venture is also hindered by the constraint of the dose to the nearby organs at risk (Yadav et al., 2021).
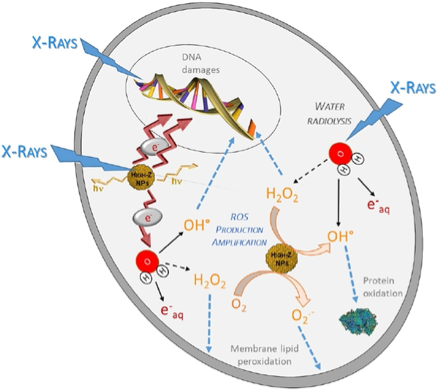
The restriction of higher doses at the tumor site became the main factor in avoiding the side effects of ionizing radiation in normal tissues. An alternative way to overcome the problem is by implementing radiosensitizer, which aims to increase the absorbed dose at the accumulated high atomic number of materials in cancerous cells only while sparing the normal tissues surrounding it. Application of radiosensitizers may improve the efficacy significance of these advanced methods over conventional ones. Radiosensitization is a method to enhance the dose deposition to the targeted treatment volume while sparing the surrounding healthy tissues. The strategies to radiosensitize the treatment site for better tumoricidal effect can be divided into three: a) reversal of radiation resistance of tumor cells, b) radioprotection of the surrounding healthy tissue, or c) radiosensitization of the tumor cells (Kwatra et al., 2013). This review will encompass the third method, radiosensitizing the tumor cells using high atomic number (Z) nanoparticles (NPs), specifically gold NPs. Figure 1 present radiosensitization mechanism of x-ray and high Z NPs that results in generation of different reactive oxygen species (ROS) production through radiolysis water molecule. Consequence of cell death such as (e.g. apoptosis, necrosis, mitotic cell death, autophagy, and permanent cell cycle arrest). With metallic NPs amplified production of ROS and secondary electron manifest radiation enhancement within targeted cancer area.
Metallic Nanoparticles as Radiosensitizers
Recently, many studies have investigated a new method for increasing optimal radiobiological impact on cancer cells by applying radiosensitizer. The application of radiosensitizer seems to be a promising technique in enhancing DNA damage and cell death in tumors and elevating radiotherapy efficacy. The mechanism behind the radiosensitization effect was correlated to the synergistic performance of each NP component, where the combination has allowed for more efficient energy transfer between the materials and facilitated the generations of secondary radiations. However, the physical enhancements further resulted in more significant ROS generation within the biological medium, damaging the intercellular components, particularly mitochondria (H. Liu et al., 2020).
The first attempt was by Matsudaira et al., 1980, using iodine as a radio-enhancer element. Nevertheless, the achievement in nanotechnology enables the production of nano-sized metal with unique characteristics such as biocompatible, easy to synthesize, high surface ratio, and tuneable. The radiosensitizing agents can be fabricated in multiple forms, such as nanoparticles (NPs) or organic and inorganic molecules, with more flexibility in functionalization and personalization. This is especially important in the future, where personalized medicine is becoming more prominent (Scott et al., 2021). This approach may be more viable for developing countries that already possess conventional radiotherapy equipment but cannot afford to have the latest advanced ones. In addition, of course, this will undeniably benefit the patients, as they will have a better chance to get improved cancer management at a considerably lower expected treatment cost.
NPs have been studied extensively in the past decade as therapeutic agents due to their unique pharmacokinetics and the simplicity of their fabrication and functionalization methods. However, only a few NPs therapeutic agents have been developed using high-atomic number (Z) metallic materials to harvest their high interaction cross-section properties, such as gold (Z=79) and hafnium (Z=72) NPs. The number of studies that report the radiosensitization effect of high-Z metallic NPs has considerably increased in past years and some of this efforts have been translated towards clinical application.
Conclusion
Metallic radiosensitizers represent a transformative approach in radiotherapy treatments, harnessing the power of advanced materials in cancer therapy. Novel metallic NPs combined with radiotherapy continue to be a groundbreaking approach in cancer treatment. Persistent research on exploring and leveraging the unique properties of these materials will significantly enhance the precision and effectiveness of radiotherapy.
References
- Rosenberg, S.A., Henke, L.E., Shaverdian, N., Mittauer, K., Wojcieszynski, A.P., Hullett, C.R., Kamrava, M., Lamb, J., Cao, M., Green, O.L. and Kashani, R., 2019. A multi-institutional experience of MR-guided liver stereotactic body radiation therapy. Advances in radiation oncology, 4(1), pp.142-149.
- Yadav, P., Kuczmarska-Haas, A., Musunuru, H.B., Witt, J., Blitzer, G., Mahler, P. and Bassetti, M.F., 2021. Evaluating dose constraints for radiation induced liver damage following magnetic resonance image guided Stereotactic Body radiotherapy. Physics and Imaging in Radiation Oncology, 17, pp.91-94.
- Kwatra, D., Venugopal, A. and Anant, S., 2013. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Translational Cancer Research, 2(4).
- Pinel, S., Thomas, N., Boura, C. and Barberi-Heyob, M., 2019. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Advanced drug delivery reviews, 138, pp.344-357.
- Liu, H., Cheng, R., Dong, X., Zhu, S., Zhou, R., Yan, L., Zhang, C., Wang, Q., Gu, Z. and Zhao, Y., 2020. BiO2–x nanosheets as Radiosensitizers with Catalase-Like activity for Hypoxia Alleviation and Enhancement of the Radiotherapy of tumors. Inorganic Chemistry, 59(6), pp.3482-3493.
- Matsudaira, H., Ueno, A.M. and Furuno, I., 1980. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to γ rays. Radiation research, 84(1), pp.144-148.
- Scott, J.G., Sedor, G., Scarborough, J.A., Kattan, M.W., Peacock, J., Grass, G.D., Mellon, E.A., Thapa, R., Schell, M., Waller, A. and Poppen, S., 2021. Personalizing radiotherapy prescription dose using genomic markers of radiosensitivity and normal tissue toxicity in NSCLC. Journal of Thoracic Oncology, 16(3), pp.428-438.
- Schuemann, J., Bagley, A.F., Berbeco, R., Bromma, K., Butterworth, K.T., Byrne, H.L., Chithrani, B.D., Cho, S.H., Cook, J.R., Favaudon, V. and Gholami, Y.H., 2020. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Physics in Medicine & Biology, 65(21), p.21RM02.
- Liu, T., Song, Y., Huang, Z., Pu, X., Wang, Y., Yin, G., Gou, L., Weng, J. and Meng, X., 2021. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids and Surfaces B: Biointerfaces, 207, p.112023.
- Zhang, Y., Han, X., Liu, Y., Wang, S., Han, X. and Cheng, C., 2022. Research progress on nano-sensitizers for enhancing the effects of radiotherapy. Materials Advances, 3(9), pp.3709-3725.
- [s1] [s2] Abdollahi, B.B., Ghorbani, M., Hamishehkar, H., Malekzadeh, R. and Farajollahi, A., 2022. Synthesis and characterization of actively HER-2 Targeted Fe3O4@ Au nanoparticles for molecular radiosensitization of breast cancer. BioImpacts: BI, 13(1), p.17.
- Tremi, I., Spyratou, E., Souli, M., Efstathopoulos, E.P., Makropoulou, M., Georgakilas, A.G. and Sihver, L., 2021. Requirements for designing an effective metallic nanoparticle (NP)-boosted radiation therapy (RT). Cancers, 13(13), p.3185.
- Choi, J., Kim, G., Cho, S.B. and Im, H.J., 2020. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. Journal of Nanobiotechnology, 18, pp.1-23.
- Dubey, P., Gupta, R., Mishra, A., Kumar, V., Bhadauria, S. and Bhatt, M.L.B., 2022. Evaluation of correlation between CD44, radiotherapy response, and survival rate in patients with advanced stage of head and neck squamous cell carcinoma (HNSCC). Cancer Medicine, 11(9), pp.1937-1947.
